Technology
EnTiCE®
Bispecific T cell engagers (TCEs) are transforming the treatment of solid tumors
While TCEs are coming of age, their impact in solid tumors has been limited by a lack of suitable targets.
Dark Antigens® are ideally suited to overcome this challenge and unlock the full potential of TCEs for patients with solid tumors

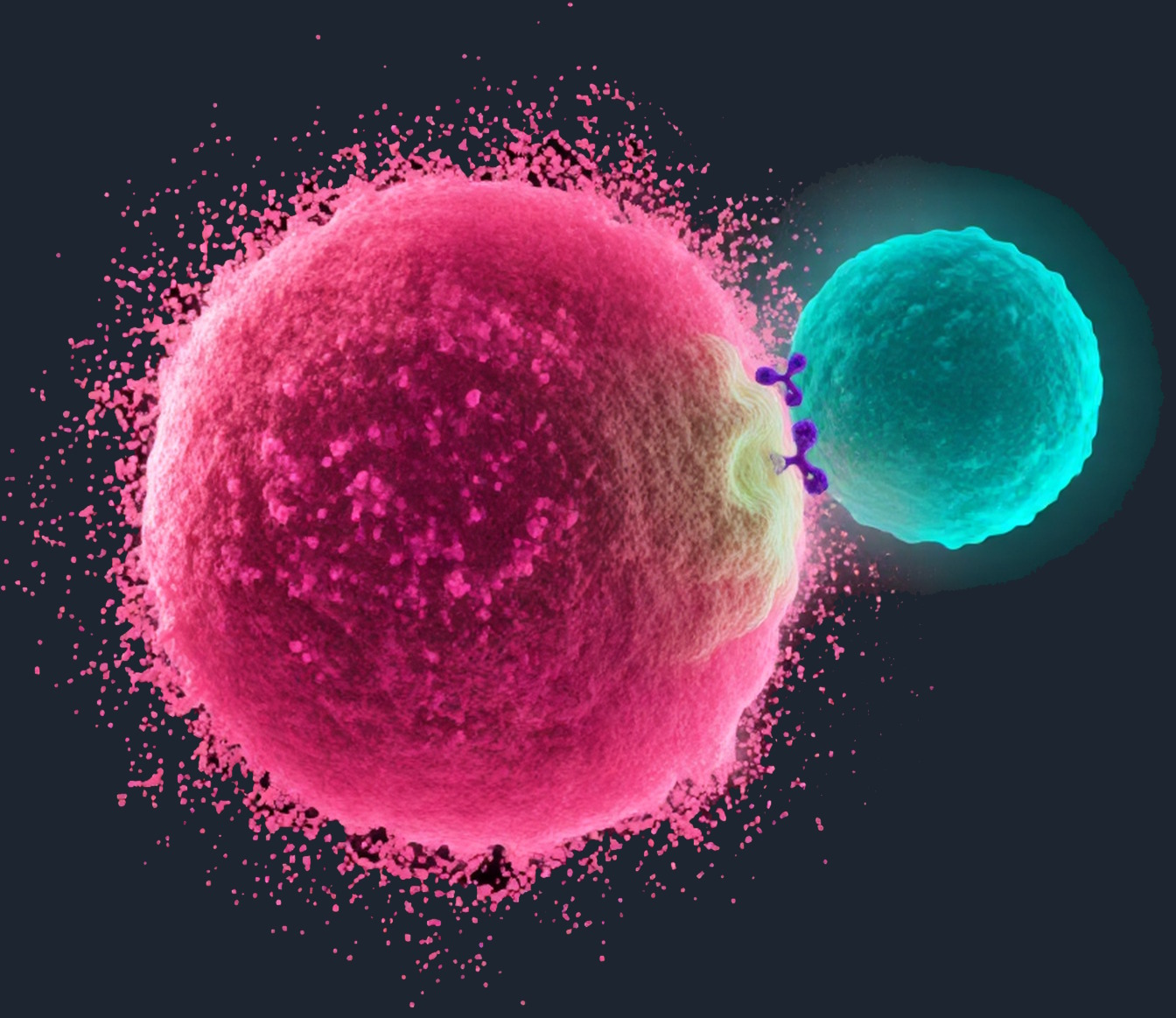
Unprecedented clinical benefit
Bispecific T cell engagers (TCEs) redirect a patient’s own T cells to recognize and kill tumor cells. Their clinical potential is now proven, exemplified by the regulatory approvals of tarlatamab in small-cell lung cancer and tebentafusp in uveal melanoma. These landmark approvals demonstrate the power of TCEs to deliver unprecedented clinical benefit for patients with solid tumors.
Discovering new targets
The broader impact of TCEs in solid tumors has been constrained by a lack of suitable targets, limiting their applicability to only a minority of patients.
Expanding the impact of TCEs requires the discovery of new targets that are:
- Highly cancer-specific with minimal expression in normal tissues – a stricter requirement than for other treatment modalities such as ADCs or vaccines.
- Shared across broad populations of patients to maximize clinical impact.
- Homogenously expressed within tumors - a key predictor of clinical efficacy.
Maximizing therapeutic potential
Dark Antigens® are ideally suited to meet these requirements, unlocking the transformative impact of TCEs for solid tumor patients. We are advancing a pipeline of first-in-class TCEs targeting Dark Antigens®, with the goal of providing durable benefit for broad populations of cancer patients.
In parallel with our antigen discovery engine, EDAPT®, we built the EnTiCE® platform so that we can develop first-in-class TCEs targeting novel cancer antigens.
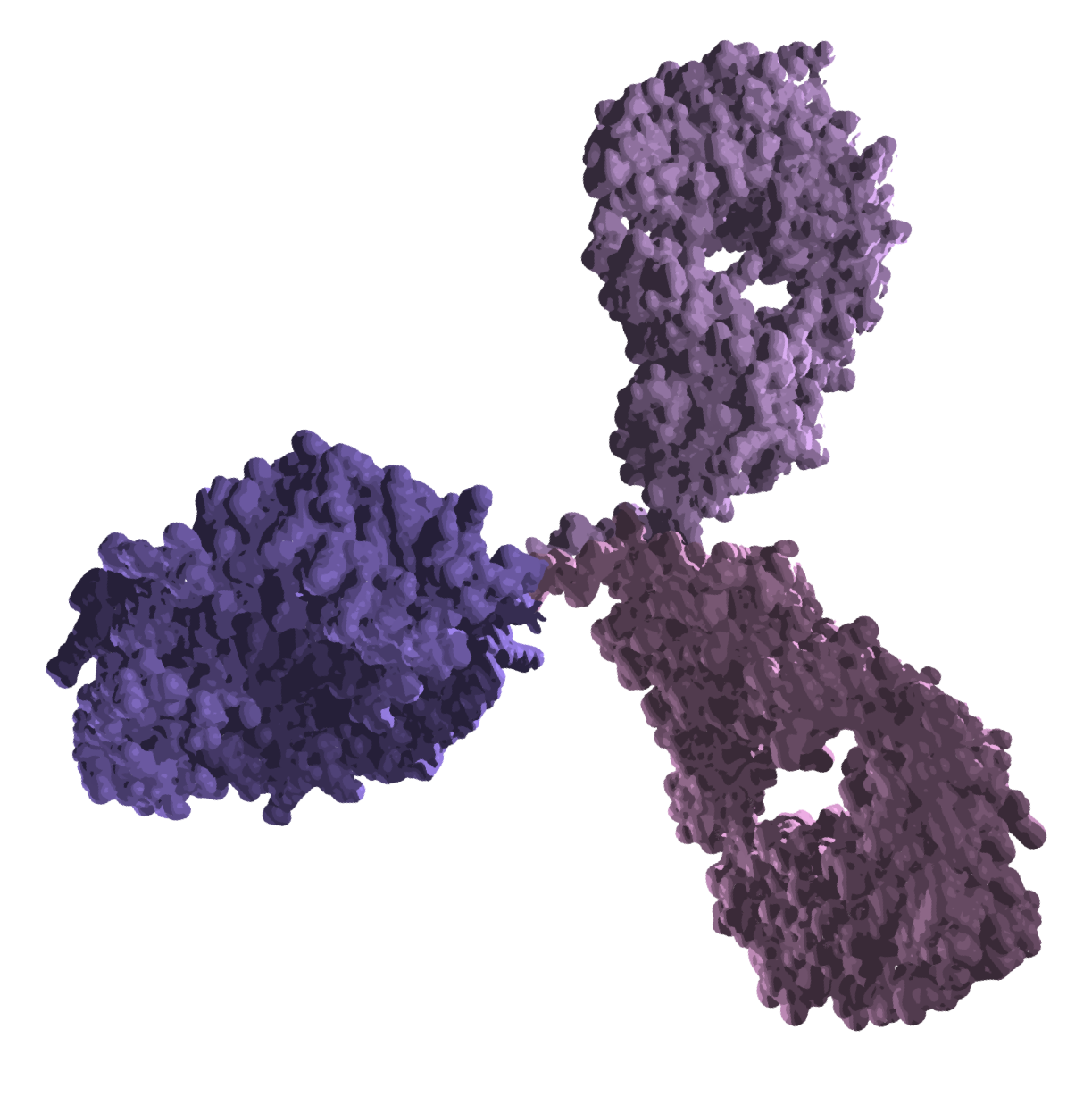
High-affinity tumor antigen binder
Tumor binding domain is engineered for high affinity to the target antigen, driving maximal clinical efficacy
Proprietary scaffold engineering to optimize the immune synapse for any binding moiety
Anti-CD3 to engage T cells with optimal kinetics
Anti-CD3 domain with kinetics for optimal T cell activation, driving powerful killing of tumor cells, while minimizing the risk of harmful over-activation and cytokine release
Silenced Fc backbone to extend
half-life
Fc domain engineered to minimize immune effector functions while prolonging systemic exposure, supporting maximal clinical benefit and patient-friendly dosing
EnTiCE® TCEs have best-in-class properties to maximize patient benefit
Every aspect of EnTiCE® molecules has been designed to have best-in-class properties to enhance therapeutic benefit for cancer patients.
To maximize clinical efficacy, we created a versatile protein engineering toolkit that allows rapid refinement of the molecular format to achieve an optimal immune synapse for any tumor antigen binder.
The EnTiCE® platform can be optimized for any class of antigen, including peptides presented by HLA molecules or cell surface proteins.
Learn about our innovative platforms
Discover how our science is driving the development of first-in-class immunotherapies designed to transform patient care.